GLP-1 agonists for weight loss - A comprehensive review
New medications like Ozempic, Wegovy, and Mounjaro (GLP-1 receptor agonists) are being hailed as game-changers for weight loss and metabolic health. Whilst these drugs can help with serious health issues, appetite, blood sugar, and weight management, they are not without significant risks! Let’s unpack the research!
What are GLP-1 medications?
GLP-1 (glucagon-like peptide-1) is a hormone naturally secreted by the gut in response to eating, particularly upon ingestion of glucose and fat (Lim & Brubaker, 2006). In a healthy system, GLP-1 helps regulate blood sugar, hunger and digestion by signalling the pancreas to release insulin after meals, inhibiting glucagon secretion to prevent excess glucose production by the liver, slowing gastric emptying to promote satiety, and communicating with the brain to support energy balance (Hammad, Patel, Magdy, & Eid, 2025). Essentially, GLP-1 acts as a metabolic “brake,” preventing overeating and helping the body use energy efficiently.
GLP-1 receptor agonists (such as Ozempic® and Wegovy®) are pharmaceutical versions of this natural hormone designed to resist rapid degradation in the body, allowing them to remain active longer and provide consistent therapeutic effects (Zhang et al., 2025). These medications mimic the effects of GLP-1 by enhancing insulin secretion in a glucose-dependent manner, suppressing glucagon release, slowing gastric emptying, and reducing appetite, and have proven effective for managing type 2 diabetes and obesity (Hammad et al., 2025; Yao et al., 2024).
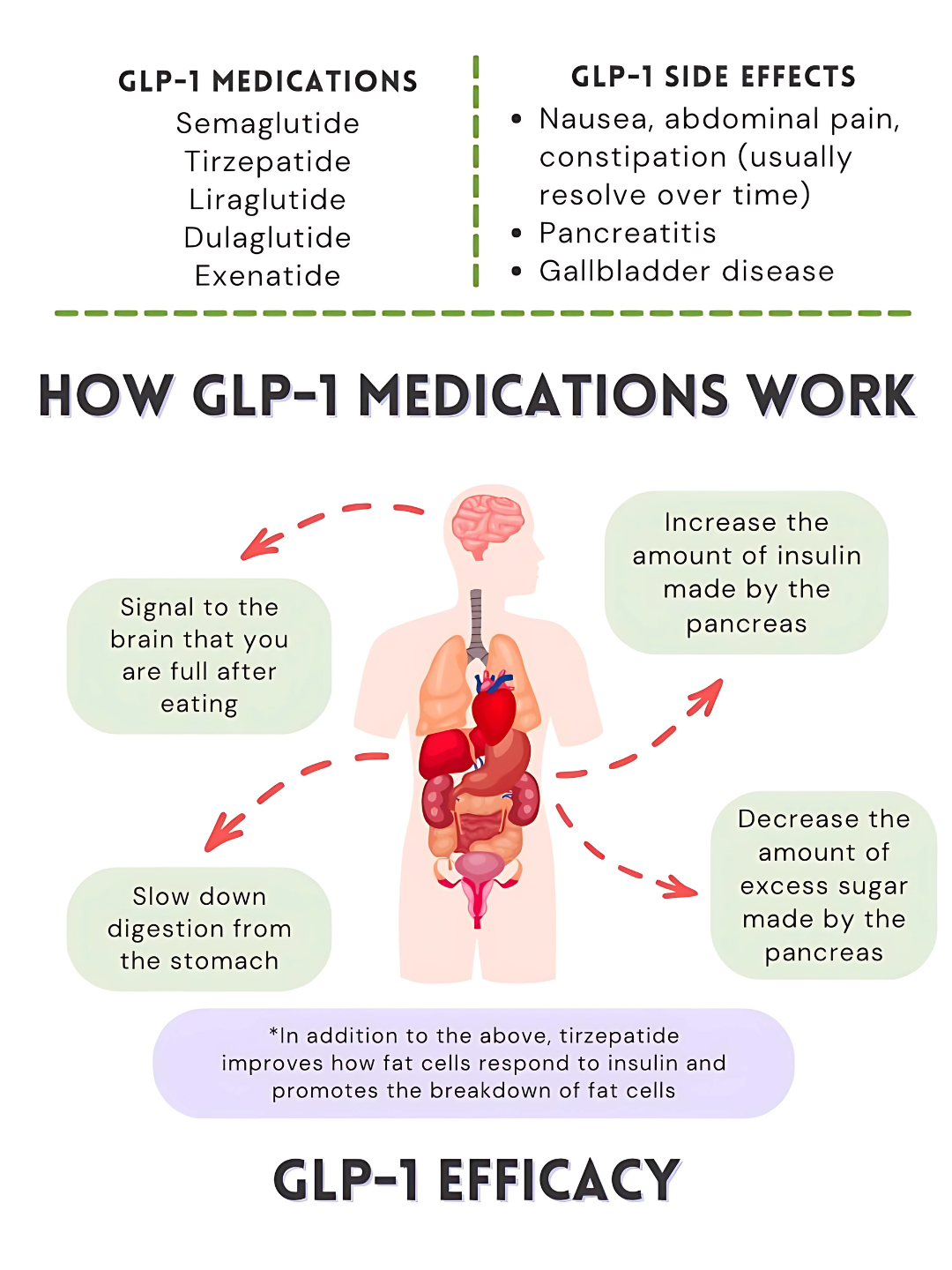
How GLP-1 manages blood sugar, hunger signals and energy balance
Do these medications work?
GLP-1 receptor agonists have demonstrated significant efficacy in managing type 2 diabetes, obesity, and chronic kidney disease (CKD). In patients with type 2 diabetes, these medications effectively reduce glycated hemoglobin (HbA1c) levels and promote weight loss, contributing to improved glycemic control and reduced cardiovascular risk (Yao et al., 2024).
In the context of obesity, GLP-1 receptor agonists aid in weight management by reducing appetite and food intake, leading to clinically significant weight loss (Son & Lim, 2024). This weight reduction is particularly beneficial for individuals with type 2 diabetes, as it can enhance insulin sensitivity and glycemic control (Son & Lim, 2024).
GLP-1 receptor agonists have also demonstrated protective effects on kidney function. Clinical studies indicate that these medications can slow the progression of kidney disease in patients with type 2 diabetes and CKD, reducing the risk of end-stage renal disease and related complications (Bae, 2025). Semaglutide, in particular, has demonstrated benefits in improving kidney function and reducing adverse renal outcomes (Bae, 2025).
Overall, GLP-1 receptor agonists represent a multifaceted therapeutic option, offering benefits that extend beyond glycemic control to include weight loss, cardiovascular and renal protection (Yao et al., 2024; Agarwal et al., 2024). So yes - they work for their intended purpose!
Figure 1. GLP-1 Medications: What You Need to Know (infographic). Reprinted from Medidex Connect by S. Webb (PharmD), January 26, 2025. Adapted with permission.
Why might the GLP-1 system be impaired in the first place?
So how does someone’s GLP-1 system become impaired? What are the underlying causes of the pathogenesis of type 2 diabetes, obesity and hunger signal dysregulation? Below are several factors that may affect the body’s natural GLP-1 production or its receptor signalling.
Gut Dysbiosis: An imbalance of beneficial vs. harmful bacteria can influence GLP-1 release and it’s signalling pathways (Zeng et al., 2023)
Liver Dysfunction: Fatty liver or inflammation disrupts glucose metabolism, potentially affecting insulin sensitivity (Jung et al., 2024)
Toxic Load: Chronic exposure to environmental toxins or accumulated metabolic waste stresses the gut-liver axis, impairing hormone production (Chiu et al., 2020).
Chronic Inflammation: Low-grade systemic inflammation, common in obesity and metabolic syndrome, can influence metabolic pathways, including those involving GLP-1 (Monteiro & Azevedo, 2010)
Diet High in Saturated Fat and Processed Foods: Saturated fats reduce membrane fluidity and lead to JNK activation, contributing to insulin resistance and inflammatory mediators (Holzer et al., 2011), whilst ultra-processed foods promote inflammation and insulin resistance, further influencing GLP-1 signalling (Figueiredo et al., 2017; Thombare et al., 2017).
Chronic Stress: Persistent stress can influence metabolic processes, potentially affecting hormone secretion (Kivimäki et al., 2023)
Together, these factors can explain why some people struggle with appetite control, blood sugar spikes, and weight gain, even if they are eating reasonable portions. Most people don’t realise that a long-term diet high in saturated fats, ultra-processed foods, and environmental toxins, especially when combined with stress and unhealthy eating habits, does more than just add extra weight. Over time, it disrupts how our cells function, damages tissues, and sets the stage for chronic disease.
Why Many People Are Turning to GLP-1 Medications
With impaired GLP-1 signalling so common, many people are turning to medications like Ozempic, Wegovy, and Mounjaro to help manage appetite, blood sugar, and weight. This is particularly relevant in Australia, where metabolic health challenges are widespread:
Approximately 1.3 million Australians are living with diabetes, a number that has nearly tripled since 2000 (Australian Institute of Health and Welfare [AIHW], 2023a).
The prevalence of non-alcoholic fatty liver disease (NAFLD) in Australia is estimated at 38.8% (Vaz et al., 2023)
In 2022–23, two-thirds of Australian adults were living with overweight (34.0%) or obesity (31.7%) (AIHW, 2023b).
GLP-1 medications mimic the effects of the natural hormone, helping to reduce appetite, slow digestion, and support weight loss. For many, this seems like an attractive solution, particularly when lifestyle changes alone have not produced the desired results. These drugs, used appropriately, can be lifesaving for some, and significantly decrease risk of type 2 diabetes, cardiovascular disease and other co-morbidities associated with obesity.
However, these medications do not address the underlying causes of impaired GLP-1 function, such as gut dysbiosis, liver stress, chronic inflammation, toxin accumulation, and poor dietary patterns. If these root causes are not addressed through nutrition and lifestyle interventions, stopping the medication may allow the underlying issues to resurface, potentially leading to a return of symptoms or worsening metabolic health.
Bottom line: Pairing GLP-1 medications with supportive nutrition and lifestyle changes (to address the root cause) may help improve long-term results and lower the risk of regaining weight after treatment.
Potential risks of taking GLP-1 medications
So, if the drugs are so effective, why not just go ahead and take them? Below is a summary of some of the potential side effects of the drugs, and why caution is advised when considering using them.
1. Digestive System side effects
Gastrointestinal Effects:
Common side effects include nausea, vomiting, diarrhea, and constipation, especially in the first few weeks. These usually improve over time. GLP-1 RAs slow gastric emptying, which can rarely raise concerns about intestinal blockage or aspiration during surgery.
Gallbladder Effects:
These drugs can increase the risk of gallstones and bile duct issues, particularly with higher doses, longer use, or significant weight loss. Monitoring is advised for those with a history of gallbladder problems.
Pancreatic Effects:
Although early reports raised concerns, most studies show no significant increase in pancreatitis or pancreatic cancer. Mild enzyme elevations can occur, but serious events are rare.
Takeaway:
Digestive and gallbladder issues are the most common side effects. Serious complications are uncommon, but careful monitoring is recommended for those with gallbladder or pancreatic history.
(Kim & Yoo, 2025)
2. Nutrient Absorption Issues & Deficiencies
When appetite is suppressed, people often eat much less, sometimes too little to meet nutritional requirements.
Some studies show that people on GLP-1 receptor agonists may develop nutritional deficiencies (e.g., of iron, vitamin B12, calcium, vitamin D) and lose lean muscle mass (Butsch et al., 2025). These biological changes could contribute to symptoms such as fatigue, reduced bone density, and possibly increased risk of fractures (Al Refaie et al., 2025).
Bottom line: When using these medications, it is essential to maintain an energy-balanced, nutrient-dense diet. Without this, deficiencies are likely to develop.
3. Muscle Wastage & Strength Loss - complete
Studies have shown that approximately 20–40% of the weight lost on GLP-1 medications may be lean mass, not just fat (Rosenstock et al., 2021; Wilding et al., 2021; Rubino et al., 2021). This loss of muscle mass can reduce metabolism, increase frailty, and impair mobility, especially in older adults (Batsis & Villareal, 2018; Chen et al., 2020). Research highlights that this muscle loss not only makes weight regain more likely but also diminishes long-term strength and function (Garvey et al., 2022).
Additionally, appetite suppression may place the body in a mild stress state. Cortisol and other stress hormones can rise when the body perceives an “energy deficit,” which may further contribute to muscle breakdown, fatigue, or lowered immunity (Ravussin & Ryan, 2018).
Bottom line: Without structured resistance training and adequate caloric/nutritional intake, patients risk sacrificing strength for short-term weight reduction.
5. Weight Regain
Weight regain after discontinuing GLP-1 receptor agonists (GLP-1 RAs) is common and often rapid due to metabolic adaptation, persistent energy imbalances, and unresolved behavioral factors. Even when lifestyle interventions are maintained, lowered resting energy expenditure following cessation can increase the risk of regaining lost weight (Ravussin & Ryan, 2018; Wilding et al., 2021). Clinical studies consistently show that many patients regain a substantial portion, or even all of the weight they initially lost within months of stopping therapy (Wilding et al., 2022; Rubino et al., 2021). Consequently, addressing underlying eating behaviours, stress management, and lifestyle habits is critical. Integrating medical weight-loss therapies with behavioural interventions, nutritional counselling, or coaching can help sustain long-term weight management and prevent rapid weight regain.
Bottom line: Without addressing the underlying relationship with food and lifestyle, weight regain is highly likely once GLP-1 medications are stopped. It might be advisable to combine medical treatment with counselling or behavioural therapy to support lasting transformation.
6. Toxic Load from Rapid Fat Loss
Fat tissue acts as a storage site for fat-soluble pollutants and toxins (Heindel et al., 2017). When fat breaks down quickly, these compounds are released into circulation.
This may temporarily overwhelm the liver, kidneys, and other detoxification pathways. If elimination is further impaired (e.g., by constipation), toxins may redistribute to other organs, creating additional health risks (Neeland et al., 2019).
Bottom line: Weight loss should ideally be gradual and supported with nutritional and detoxification strategies.
7. Thyroid Cancer Risk
Animal studies raised concern about thyroid tumours in response to GLP-1 drugs. Human data remain mixed and inconclusive.
A large Scandinavian study found no increased thyroid cancer risk (Pottegård et al., 2025). However, a French cohort reported a 58% higher risk after 1–3 years of use (Bezin et al., 2023).
Bottom line: Following the principle of “first, do no harm,” caution is warranted until definitive, long-term data is made available.
8. Other Side Effects Reported in the Literature
Beyond those already discussed in detail above, GLP-1 receptor agonists have been associated with a wide range of additional side effects:
Common: Headache, fatigue, dizziness, injection-site reactions, mild hypoglycaemia (when combined with insulin or sulfonylureas).
Gastrointestinal: Delayed gastric emptying (problematic during surgery or anesthesia), gastroesophageal reflux, abdominal pain.
Hepatobiliary: Gallbladder disease, gallstones, biliary colic.
Pancreatic: Rare cases of pancreatitis, pancreatic enzyme elevations.
Renal: Dehydration and acute kidney injury (often secondary to vomiting or diarrhea).
Cardiovascular: Mild increases in heart rate, rare arrhythmias.
Neurological: Rare reports of altered taste, mild cognitive complaints.
Immunological: Rare hypersensitivity reactions.
Emerging / Theoretical: Alterations to the gut microbiome, long-term sarcopenia risk, and possible psychiatric side effects such as increased anxiety or depression in sensitive individuals.
(Manne-Goehler & Franco, 2025)
The Naturopathic “Do No Harm” Approach
In naturopathic medicine, primum non nocere—“first, do no harm”—means beginning with the safest, least invasive options before turning to stronger interventions. When it comes to GLP-1 medications, this principle guides us to carefully investigate the root causes of weight gain and to screen for potential risks before taking the GLP-1 drug.
Identifying Underlying Issues That Could Be Resolved Without Medication
Many drivers of weight gain can be uncovered and addressed through targeted testing, often reducing or even removing the need for pharmaceutical support:
Basic blood work (CBC, liver and kidney function): Screens for inflammation, hidden organ stress, or deficiencies that may affect metabolism and energy (Calder et al., 2017).
Blood sugar regulation (fasting glucose, HbA1c, fasting insulin, HOMA-IR): Detects insulin resistance or prediabetes, which can sometimes be corrected with nutrition, exercise, and stress support (Cornier et al., 2011).
Thyroid panel (TSH, Free T4, Free T3, ± antibodies): Evaluates whether thyroid dysfunction is contributing to weight changes (Taylor et al., 2018).
Hormone panel (cortisol, estrogen, progesterone, testosterone, DHEA): Identifies imbalances in stress or sex hormones that may drive weight gain or resistance to fat loss (Pasquali, 2012).
Gut health testing (stool analysis, SIBO breath test, gut inflammation markers): Assesses digestive function, nutrient absorption, and microbiome balance, all of which affect weight and cravings (Zmora et al., 2019).
Body composition analysis (lean mass vs. fat mass): Helps distinguish fat-related weight gain from muscle loss or sarcopenia, guiding a more tailored approach (Prado & Heymsfield, 2014).
Screening for Risk Factors Before Starting GLP-1 Medication
Functional and standard tests can identify whether a patient carries higher risk for known drug side effects, allowing precautions:
Gallbladder and liver markers (liver enzymes, ultrasound if indicated): Since GLP-1 drugs can increase risk of gallstones and gallbladder disease, pre-existing issues should be ruled out (Wilding et al., 2021; Nauck et al., 2016).
Pancreatic enzymes (amylase, lipase): Screens for silent pancreatic stress, important given the risk of pancreatitis with GLP-1s (Nauck et al., 2016).
Full thyroid panel ± calcitonin: A full thyroid panel, with or without calcitonin, ensures baseline thyroid health and identifies any red flags, as concerns have been raised about thyroid C-cell tumor risk in rodent studies and the theoretical risk in humans (Aroda et al., 2017).
Lipid profile (cholesterol, triglycerides): Evaluates cardiovascular risk, which may influence whether these medications are the safest option (Garber et al., 2020).
Nutrient status (vitamin B12, vitamin D, iron, calcium): Identifies deficiencies that could be worsened by appetite suppression on the medication (Mullin et al., 2020; Thakkar et al., 2015).
This personalised assessment honours the naturopathic principles of:
First, do no harm – start with the safest, least invasive strategies.
Identify and treat the cause – uncover and address the true drivers of weight gain.
Support the whole person – recognise that weight is not just about calories, but about the interconnected balance of hormones, organs, and lifestyle (Cornier et al., 2011).
Solutions: Evidence-Based, Drug-Sparing Ways to Improve Insulin Sensitivity and Lose Fat
In light of all of the risks associated with the medication, what are the other options? Are there actually solutions to resolving the ROOT CAUSE of blood sugar dysregulation, excess fat storage and insatiable hunger? There sure are!
1) Make plants the foundation (especially lower-fat, higher-fibre)
A plant-predominant, lower-fat dietary pattern consistently improves insulin sensitivity, glycemic control, and cardiometabolic risk, often beyond what weight change alone would predict. Randomised trials of low-fat vegan or plant-forward diets show improved insulin sensitivity, β-cell function, and HbA1c compared with conventional diets, alongside favourable changes in body composition (Kahleová et al., 2018; Kahleová et al., 2020). High-fiber, minimally processed plant foods also nourish a healthier, more diverse gut microbiome, which is linked to lower inflammatory tone and better metabolic regulation (Singh et al., 2017; Sonnenburg & Sonnenburg, 2019).
Practical takeaways: Build meals around fruits, vegetables, legumes, whole grains and minimal nuts/seeds; keep added fats and ultra-processed foods low; aim for ≥30–40 g/day of fiber (Kahleová et al., 2020; Sonnenburg & Sonnenburg, 2019).
2) Train your metabolism with movement (resistance + aerobic)
Exercise increases insulin sensitivity through muscle-specific mechanisms even in the absence of major weight loss (Bird & Hawley, 2017). Combining resistance training with aerobic work optimises fat loss while preserving (or gaining) lean mass, critical for sustaining resting energy expenditure and preventing the “regain” trap (Villareal et al., 2017). In older adults with obesity, programs that include both resistance and aerobic training during weight reduction improve insulin sensitivity indices, and reduce visceral and inter-muscular fat compared with either mode alone (Weiss et al., 2022).
Practical takeaways: 2–3 non-consecutive days/week of resistance training (large compound movements) plus 150–300 minutes/week of moderate aerobic activity or interval work; prioritise progressive overload and adequate recovery (Weiss et al., 2022; Bird & Hawley, 2017).
3) Address stress and emotional drivers (mindfulness, CBT, and coaching)
Stress reactivity and dysregulated eating patterns undermine metabolic health. Randomised trials and meta-analyses show mindfulness-based interventions and related programs produce small-to-moderate improvements in emotional eating and modest but meaningful weight and waist reductions, with reductions in cortisol reactivity in some studies (Daubenmier et al., 2011; Carbone et al., 2022; Rogers et al., 2021; Mason et al., 2016).
Practical takeaways: Incorporate mindfulness-based stress reduction, mindful eating skills, or CBT-based coaching alongside nutrition and exercise to lock in behaviour change and protect against relapse (Rogers et al., 2021; Mason et al., 2016).
4) Hydrate strategically
Simple hydration habits can support appetite regulation and weight control. In a randomized trial, drinking ~500 mL water before each main meal enhanced 12-week weight loss beyond a hypocaloric diet alone in middle-aged and older adults (Dennis et al., 2010). Meta-analyses now support that pre-meal water loading can significantly augment weight loss and reduce daily energy intake (Pan et al., 2021).
Practical takeaways: Target regular water intake across the day and consider a pre-meal water “preload,” particularly if you’re middle-aged or older (Dennis et al., 2010; Pan et al., 2021).
5) Guard your circadian rhythm (sleep and morning light)
Short sleep and circadian misalignment impair insulin sensitivity and promote preferential visceral fat gain, even over just a few weeks (Eckel et al., 2015; Covassin et al., 2022; Leproult & Van Cauter, 2010). Morning outdoor light helps anchor circadian timing, indirectly supporting appetite and glucose regulation (Depner et al., 2018).
Practical takeaways: Aim for 7–9 hours/night, keep a consistent sleep-wake schedule, seek outdoor morning light exposure, and limit late-evening light, heavy meals, and alcohol (Covassin et al., 2022; Depner et al., 2018).
Bottom line
A plant-focussed, lower-fat, higher-fibre diet, structured resistance + aerobic training, stress-management skills (mindfulness/CBT), consistent hydration and circadian-aligned sleep/light exposure can form a potent, drug-sparing strategy to improve insulin sensitivity, reduce visceral fat, and sustain weight loss, without the risk of medications.
These interventions directly target the biological systems GLP-1 agonists attempt to influence whilst building durable habits and metabolic resilience (Kahleová et al., 2018; Kahleová et al., 2020; Villareal et al., 2017; Bird & Hawley, 2017; Rogers et al., 2021; Mason et al., 2016; Pan et al., 2021; Depner et al., 2018).
Summary
GLP-1 agonists can deliver quick results, with far less effort than changing your diet, lifestyle, exercising, getting adequate sleep and taking the time to educate yourself on your health. We all want a quick fix, and this drug seems to do it exceptionally well. However, if you are concerned about the risks, or potential weight regain after drug cessation, there ARE solutions, and other benefits to incorporating these practices into your lifestyle, such as increased energy, bowel health, balanced mood, reduced stress and increased muscle mass. The research is clear!
These drugs are not a long-lasting solution, especially if you start running into dramas with nutritional deficiencies, muscle mass loss and other side effects. There is not, and will never be any drug on the market that will RESOLVE the root cause of your health challenges. Only you can go on that journey, one day at a time.
If you’re considering these medications, I encourage you to explore thorough testing, emotional support, and nutritional strategies before or alongside your treatment. As a practitioner, there are circumstances in which I feel it would be suitable for people to take these medications for a short period of time, alongside the above natural strategies to complement the treatment. There are other times when I feel the risk factors may outweigh the benefits for some people, but this would only be discovered upon further consultation and testing.
Having said all of that, for long lasting, sustainable weight loss, I recommend seeking a clinically trained health practitioner to educate you, source appropriate testing, and support you on your journey. Please reach out if you’re looking for further support on this matter.
References
Agarwal, A., Mustafa, R., Manja, V., et al. (2024). Cardiovascular, kidney related, and weight loss effects of therapeutics for type 2 diabetes: A living systematic review and network meta-analysis. The BMJ, 390, bmj-2024-082071. https://doi.org/10.1136/bmj-2024-082071
Al Refaie, A., Baldassini, L., Mondillo, C., Gonnelli, S., Ceccarelli, E., Tarquini, R., Gonnelli, S., Gennari, L., & Caffarelli, C. (2025). Glucagon-like peptide-1 receptor agonists (GLP-1RAs) for the treatment of type 2 diabetes mellitus: Friends or foes to bone health? A narrative review of clinical studies. Endocrine, 89(1), 30-38. https://doi.org/10.1007/s12020-025-04253-4
Aroda, V. R., Henry, R. R., Han, J., Krol, E., & Mari, A. (2017). Long-term safety of GLP-1 receptor agonists: Risk of thyroid C-cell tumors. Diabetes, Obesity and Metabolism, 19(7), 940–948. https://doi.org/10.1111/dom.12916
Australian Institute of Health and Welfare. (2023a). Diabetes: Australian facts. https://www.aihw.gov.au/reports/diabetes/diabetes/contents/summary
Australian Institute of Health and Welfare. (2023b). Overweight and obesity - Australian adults. https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity/contents/summary
Bae, J. H. (2025). SGLT2 inhibitors and GLP-1 receptor agonists in diabetic kidney disease: Evolving evidence and clinical application. Diabetes & Metabolism Journal, 49(3), 386–402. https://doi.org/10.4093/dmj.2025.0220
Batsis, J. A., & Villareal, D. T. (2018). Sarcopenia and obesity. Clinical Geriatrics, 26(1), 12–18
Bezin, J., et al. (2023). GLP-1 receptor agonists and risk of thyroid cancer: A French nationwide cohort study. Diabetes Care, 46(6), 1235–1243. https://doi.org/10.2337/dc22-1923
Bird, S. R., & Hawley, J. A. (2017). Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Diabetes/Metabolism Research and Reviews, 33(5), e2871. https://doi.org/10.1002/dmrr.2871
Butsch, W. S., Sulo, S., Chang, A. T., Kim, J. A., Kerr, K. W., Williams, D. R., Hegazi, R., Panchalingam, T., Goates, S., & Heymsfield, S. B. (2025). Nutritional deficiencies and muscle loss in adults with type 2 diabetes using GLP-1 receptor agonists: A retrospective observational study. *Obesity Pillars,15, Article 100186. https://doi.org/10.1016/j.obpill.2025.100186
Calder, P. C., et al. (2017). The role of nutrition in the prevention and treatment of inflammation. European Journal of Clinical Nutrition, 71(3), 267–277. https://doi.org/10.1038/ejcn.2016.236
Carbone, S., Cioffi, I., & D’Amico, M. (2022). Mindfulness-based interventions for emotional eating and weight outcomes: A systematic review and meta-analysis. Appetite, 176, 106072. https://doi.org/10.1016/j.appet.2022.106072
Chen, L. K., et al. (2020). Sarcopenia in older adults: A review. Journal of the American Geriatrics Society, 68(4), 748–758.
Chiu, K., Warner, G., Nowak, R. A., Flaws, J. A., & Mei, W. (2020). The impact of environmental chemicals on the gut microbiome. Toxicological Sciences, 176(2), 253–284. https://doi.org/10.1093/toxsci/kfaa065
Cornier, M. A., et al. (2011). The metabolic syndrome. Endocrine Reviews, 32(1), 1–45. https://doi.org/10.1210/er.2009-0024
Covassin, N., Zhang, R., Spagnolli, S., & DeFina, L. (2022). Sleep and circadian rhythm disruption: Implications for metabolic health and obesity. Current Diabetes Reports, 22(5), 177–190. https://doi.org/10.1007/s11892-022-01474-7
Daubenmier, J., Kristeller, J., Hecht, F. M., Maninger, N., Kuwata, M., Jhaveri, K., ... & Epel, E. (2011). Mindfulness intervention for stress eating to reduce cortisol and abdominal fat in overweight and obese women: A randomized controlled trial. Obesity, 19(11), 2253–2259. https://doi.org/10.1038/oby.2011.186
Dennis, E. A., Dengo, A. L., Comber, D. L., Flack, K. D., Savla, J., Davy, K. P., & Davy, B. M. (2010). Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity, 18(2), 300–307. https://doi.org/10.1038/oby.2009.235
Depner, C. M., Stothard, E. R., & Wright, K. P., Jr. (2018). Metabolic consequences of sleep and circadian disorders. Current Diabetes Reports, 18(7), 55. https://doi.org/10.1007/s11892-018-1005-7
Eckel, R. H., Grundy, S. M., & Zimmet, P. Z. (2015). The metabolic syndrome. The Lancet, 365(9468), 1415–1428. https://doi.org/10.1016/S0140-6736(05)66378-7
Figueiredo, P. S., Inada, A. C., Marcelino, G., Cardozo, C. M. L., Freitas, K. D. C., Guimarães, R. D. C. A., Castro, A. P., Nascimento, V. A. D., & Hiane, P. A. (2017). Fatty acids consumption: The role metabolic aspects involved in obesity and its associated disorders. Nutrients, 9(10), Article 1158. https://doi.org/10.3390/nu9101158
Garber, A. J., Handelsman, Y., Grunberger, G., Einhorn, D., Abrahamson, M. J., Barzilay, J. I., ... & Mechanick, J. I. (2020). Consensus statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocrine Practice, 26(1), 107–139. https://doi.org/10.4158/CS-2019-0472
Garvey, W. T., et al. (2022). Effects of weight loss on muscle mass and function. Obesity Reviews, 23(5), e13356.
Hammad, T., Patel, S., Magdy, T., & Eid, A. H. (2025). Exploring the multifaceted roles of GLP-1 receptor agonists: A comprehensive review. Frontiers in Clinical Diabetes and Healthcare, 6, 1590530. https://doi.org/10.3389/fcdhc.2025.1590530
Holzer, R. G., Park, E.-J., Li, N., Tran, H., Chen, M., Choi, C., Solinas, G., & Karin, M. (2011). Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell, 147(1), 173–184. https://doi.org/10.1016/j.cell.2011.08.034
Jung, I., Koo, D.-J., & Lee, W.-Y. (2024). Insulin resistance, non-alcoholic fatty liver disease, and type 2 diabetes mellitus: Clinical and experimental perspective. Diabetes & Metabolism Journal, 48(3), 327–339. https://doi.org/10.4093/dmj.2023.0350
Kahleová, H., Tura, A., Hill, M., Holubkov, R., & Barnard, N. D. (2018). A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: A randomized clinical trial. Nutrients, 10(2), 189. https://doi.org/10.3390/nu10020189
Kahleová, H., Petersen, K. S., Holubkov, R., & Barnard, N. D. (2020). Effect of a plant-based dietary intervention on cardiometabolic risk factors: A randomized clinical trial. Journal of the American Heart Association, 9(16), e016179. https://doi.org/10.1161/JAHA.120.016179
Kim, J. A., & Yoo, H. J. (2025). Exploring the side effects of GLP-1 receptor agonists: To ensure its optimal positioning. Diabetes & Metabolism Journal, 49(4), 525–541. https://doi.org/10.4093/dmj.2025.0242
Kivimäki, M., Bartolomucci, A., & Kawachi, I. (2023). The multiple roles of life stress in metabolic disorders. Nature Reviews Endocrinology, 19(1), 10–27. https://doi.org/10.1038/s41574-022-00746-8
Leproult, R., & Van Cauter, E. (2010). Role of sleep and sleep loss in hormonal release and metabolism. Endocrine Development, 17, 11–21. https://doi.org/10.1159/000262524
Lim, G. E., & Brubaker, P. L. (2006). Glucagon-like peptide-1 secretion by the L cell: The view from within. Diabetes, 55(Suppl. 2), S70–S77. https://doi.org/10.2337/db06-S020
Manne-Goehler, J., & Franco, J. (2025). Side effects of GLP-1 receptor agonists. The BMJ, 390, r1606. https://doi.org/10.1136/bmj.r1606BMJ
Mason, A. E., Epel, E. S., Kristeller, J., Moran, P. J., Dallman, M., Lustig, R. H., ... & Daubenmier, J. (2016). Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: Data from the SHINE randomized controlled trial. Journal of Behavioral Medicine, 39(2), 201–213. https://doi.org/10.1007/s10865-015-9670-8
Monteiro, R., & Azevedo, I. (2010). Chronic inflammation in obesity and the metabolic syndrome. Mediators of Inflammation, 2010, Article 289645. https://doi.org/10.1155/2010/289645
Mullin, G. E., Dubé, P., & Theis, D. (2020). The impact of GLP-1 receptor agonists on nutrient status. Current Opinion in Endocrinology, Diabetes, and Obesity, 27(5), 337–343. https://doi.org/10.1097/MED.0000000000000577
Neeland, I. J., Ross, R., Després, J. P., Matsuzawa, Y., Yamashita, S., Shai, I., … & Després, J. P. (2019). Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes & Endocrinology, 7(9), 715–725. https://doi.org/10.1016/S2213-8587(19)30084-1
Nauck, M. A., Quast, D. R., Wefers, J., & Meier, J. J. (2016). GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Molecular Metabolism, 46, 101102. https://doi.org/10.1016/j.molmet.2020.101102
Pan, A., Schernhammer, E. S., Sun, Q., & Hu, F. B. (2021). Water consumption and risk of overweight and obesity in adults: A systematic review and meta-analysis. Obesity Reviews, 22(4), e13158. https://doi.org/10.1111/obr.13158
Pasquali, R. (2012). Obesity and androgens: Facts and perspectives. Fertility and Sterility, 97(1), 46–52. https://doi.org/10.1016/j.fertnstert.2011.11.029
Pottegård, A., et al. (2025). GLP-1 receptor agonists and risk of thyroid cancer in Scandinavia: A multinational cohort study. BMJ, 380, e072785. https://doi.org/10.1136/bmj-2022-072785
Prado, C. M., & Heymsfield, S. B. (2014). Lean tissue imaging: A new era for nutritional assessment and intervention. The Journal of Parenteral and Enteral Nutrition, 38(8), 940–953. https://doi.org/10.1177/0148607114546826
Ravussin, E., & Ryan, A. S. (2018). Glucagon-like peptide-1 receptor agonists: Implications for weight loss and metabolic health. Obesity, 26(3), 445–453.
Rogers, J. M., Ferrari, M., Mosely, K., Lang, C., & Brennan, L. (2021). Mindfulness-based interventions for adults who are overweight or obese: A meta-analysis of physical and psychological health outcomes. Obesity Reviews, 22(2), e13146. https://doi.org/10.1111/obr.13146
Rubino, D., et al. (2021). Weight loss and body composition changes with GLP-1 receptor agonists. Diabetes, Obesity and Metabolism, 23(5), 1114–1122.
Rosenstock, J., et al. (2021). Effects of GLP-1 receptor agonists on weight loss and body composition. Diabetes Care, 44(3), 657–664.
Singh, R. K., Chang, H. W., Yan, D., Lee, K. M., Ucmak, D., Wong, K., ... & Liao, W. (2017). Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 15(1), 73. https://doi.org/10.1186/s12967-017-1175-y
Son, J. W., & Lim, S. (2024). Glucagon-like peptide-1 based therapies: A new horizon in obesity management. Endocrinology and Metabolism, 39(2), 206–221. https://doi.org/10.3803/EnM.2024.1940
Sonnenburg, J. L., & Sonnenburg, E. D. (2019). The ancestral and industrialized gut microbiota and implications for human health. Nature Reviews Microbiology, 17(6), 383–390. https://doi.org/10.1038/s41579-019-0191-8
Taylor, P. N., et al. (2018). Global epidemiology of hyperthyroidism and hypothyroidism. Nature Reviews Endocrinology, 14(5), 301–316. https://doi.org/10.1038/nrendo.2018.18
Thakkar, P., Silva, R. J., & Byram, D. (2015). Vitamin B12 deficiency in patients on GLP-1 receptor agonists. Nutrition in Clinical Practice, 30(3), 427–433. https://doi.org/10.1177/0884533615572930
Thombare, K., Ntika, S., Wang, X., & Krizhanovskii, C. (2017). Long chain saturated and unsaturated fatty acids exert opposing effects on viability and function of GLP-1-producing cells: Mechanisms of lipotoxicity. PLOS ONE, 12(5), e0177605. https://doi.org/10.1371/journal.pone.0177605
Vaz, K., Kemp, W., Majeed, A., Lubel, J., Magliano, D. J., Glenister, K. M., Bourke, L., Simmons, D., & Roberts, S. K. (2023). Non‐alcoholic fatty liver disease prevalence in Australia has risen over 15 years in conjunction with increased prevalence of obesity and reduction in healthy lifestyle. Journal of Gastroenterology and Hepatology, 38(8), 1823–1831. https://doi.org/10.1111/jgh.16314
Villareal, D. T., Aguirre, L., Gurney, A. B., Waters, D. L., Sinacore, D. R., Colombo, E., ... & Armamento-Villareal, R. (2017). Aerobic or resistance exercise, or both, in dieting obese older adults. New England Journal of Medicine, 376(20), 1943–1955. https://doi.org/10.1056/NEJMoa1616338
Weiss, E. P., Fontana, L., Villareal, D. T., & Klein, S. (2022). Exercise, weight loss, and insulin sensitivity in older adults with obesity. Obesity, 30(6), 1153–1165. https://doi.org/10.1002/oby.23457
Wilding, J. P., et al. (2021). Efficacy of GLP-1 receptor agonists in weight management. Lancet Diabetes & Endocrinology, 9(5), 313–324.
Wilding, J. P. H., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., ... & Kushner, R. F. (2021). Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine, 384, 989–1002. https://doi.org/10.1056/NEJMoa2032183
Wilding, J. P. H., et al. (2022). Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes, Obesity and Metabolism, 24(8), 1553–1564. https://doi.org/10.1111/dom.14725
Yao, H., Zhang, A., Li, D., et al. (2024). Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. BMJ, 384, e076410. https://doi.org/10.1136/bmj-2023-076410
Zeng, Y., Wu, Y., Zhang, Q., & Xiao, X. (2023). Crosstalk between glucagon-like peptide 1 and gut microbiota in metabolic diseases. mBio, 15(1), e02032-23. https://doi.org/10.1128/mbio.02032-23
Zhang, L., Li, Y., Chen, X., Wang, H., & Zhao, J. (2025). Pharmacovigilance analysis of neurological adverse events associated with GLP-1 receptor agonists. Scientific Reports, 15(1), 18063. https://doi.org/10.1038/s41598-025-01206-9
Zmora, N., et al. (2019). Taking it slow: A practical guide to the gut microbiome. Gut, 68(1), 1–9. https://doi.org/10.1136/gutjnl-2018-317293
Image reference list:
Webb, S. (2025, January 26). What you need to know: GLP-1 medications infographic. Medidex Connect. Retrieved from https://medidex.chat/what-you-need-to-know-glp-1-medications-infographic/