Thinking Twice About GLP-1 Agonists? Here’s What You Should Know
New medications like Ozempic, Wegovy, and Mounjaro (GLP-1 receptor agonists) are being hailed as game-changers for weight loss and metabolic health. Whilst these drugs can help with serious health issues, appetite, blood sugar, and weight management, they are not without significant risks! Let’s unpack the research!
What are GLP-1 medications?
GLP-1 (glucagon-like peptide-1) is a hormone naturally secreted by the gut in response to eating, particularly upon ingestion of glucose and fat (Lim & Brubaker, 2006). In a healthy system, GLP-1 helps regulate blood sugar, hunger and digestion by signalling the pancreas to release insulin after meals, inhibiting glucagon secretion to prevent excess glucose production by the liver, slowing gastric emptying to promote satiety, and communicating with the brain to support energy balance (Hammad, Patel, Magdy, & Eid, 2025). Essentially, GLP-1 acts as a metabolic “brake,” preventing overeating and helping the body use energy efficiently.
GLP-1 receptor agonists (such as Ozempic® and Wegovy®) are pharmaceutical versions of this natural hormone designed to resist rapid degradation in the body, allowing them to remain active longer and provide consistent therapeutic effects (Zhang et al., 2025). These medications mimic the effects of GLP-1 by enhancing insulin secretion in a glucose-dependent manner, suppressing glucagon release, slowing gastric emptying, and reducing appetite, and have proven effective for managing type 2 diabetes and obesity (Hammad et al., 2025; Yao et al., 2024).
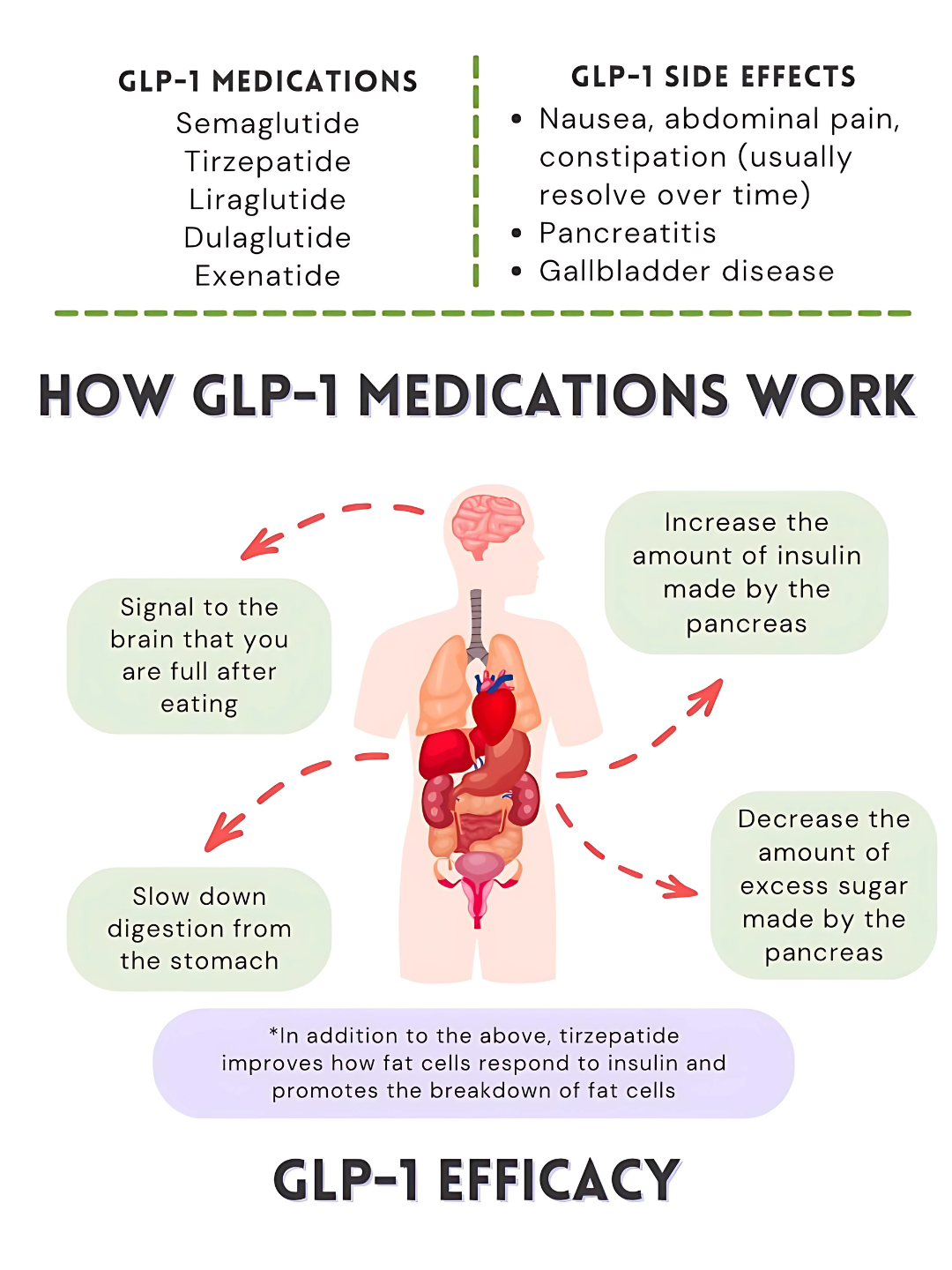
How GLP-1 manages blood sugar, hunger signals and energy balance
Do these medications work?
GLP-1 receptor agonists have demonstrated significant efficacy in managing type 2 diabetes, obesity, and chronic kidney disease (CKD). In patients with type 2 diabetes, these medications effectively reduce glycated hemoglobin (HbA1c) levels and promote weight loss, contributing to improved glycemic control and reduced cardiovascular risk (Yao et al., 2024).
In the context of obesity, GLP-1 receptor agonists aid in weight management by reducing appetite and food intake, leading to clinically significant weight loss (Son & Lim, 2024). This weight reduction is particularly beneficial for individuals with type 2 diabetes, as it can enhance insulin sensitivity and glycemic control (Son & Lim, 2024).
GLP-1 receptor agonists have also demonstrated protective effects on kidney function. Clinical studies indicate that these medications can slow the progression of kidney disease in patients with type 2 diabetes and CKD, reducing the risk of end-stage renal disease and related complications (Bae, 2025). Semaglutide, in particular, has demonstrated benefits in improving kidney function and reducing adverse renal outcomes (Bae, 2025).
Overall, GLP-1 receptor agonists represent a multifaceted therapeutic option, offering benefits that extend beyond glycemic control to include weight loss, cardiovascular and renal protection (Yao et al., 2024; Agarwal et al., 2024). So yes - they work for their intended purpose!
Figure 1. GLP-1 Medications: What You Need to Know (infographic). Reprinted from Medidex Connect by S. Webb (PharmD), January 26, 2025. Adapted with permission.
Why might the GLP-1 system be impaired in the first place?
So how does someone’s GLP-1 system become impaired? What are the underlying causes of the pathogenesis of type 2 diabetes, obesity and hunger signal dysregulation? Below are several factors that may affect the body’s natural GLP-1 production or its receptor signalling.
Gut Dysbiosis: An imbalance of beneficial vs. harmful bacteria can influence GLP-1 release and it’s signalling pathways (Zeng et al., 2023)
Liver Dysfunction: Fatty liver or inflammation disrupts glucose metabolism, potentially affecting insulin sensitivity (Jung et al., 2024)
Toxic Load: Chronic exposure to environmental toxins or accumulated metabolic waste stresses the gut-liver axis, impairing hormone production (Chiu et al., 2020).
Chronic Inflammation: Low-grade systemic inflammation, common in obesity and metabolic syndrome, can influence metabolic pathways, including those involving GLP-1 (Monteiro & Azevedo, 2010)
Diet High in Saturated Fat and Processed Foods: Saturated fats reduce membrane fluidity and lead to JNK activation, contributing to insulin resistance and inflammatory mediators (Holzer et al., 2011), whilst ultra-processed foods promote inflammation and insulin resistance, further influencing GLP-1 signalling (Figueiredo et al., 2017; Thombare et al., 2017).
Chronic Stress: Persistent stress can influence metabolic processes, potentially affecting hormone secretion (Kivimäki et al., 2023)
Together, these factors can explain why some people struggle with appetite control, blood sugar spikes, and weight gain, even if they are eating reasonable portions. Most people don’t realise that a long-term diet high in saturated fats, ultra-processed foods, and environmental toxins, especially when combined with stress and unhealthy eating habits, does more than just add extra weight. Over time, it disrupts how our cells function, damages tissues, and sets the stage for chronic disease.
Why Many People Are Turning to GLP-1 Medications
With impaired GLP-1 signalling so common, many people are turning to medications like Ozempic, Wegovy, and Mounjaro to help manage appetite, blood sugar, and weight. This is particularly relevant in Australia, where metabolic health challenges are widespread:
Approximately 1.3 million Australians are living with diabetes, a number that has nearly tripled since 2000 (Australian Institute of Health and Welfare [AIHW], 2023a).
The prevalence of non-alcoholic fatty liver disease (NAFLD) in Australia is estimated at 38.8% (Vaz et al., 2023)
In 2022–23, two-thirds of Australian adults were living with overweight (34.0%) or obesity (31.7%) (AIHW, 2023b).
GLP-1 medications mimic the effects of the natural hormone, helping to reduce appetite, slow digestion, and support weight loss. For many, this seems like an attractive solution, particularly when lifestyle changes alone have not produced the desired results. These drugs, used appropriately, can be lifesaving for some, and significantly decrease risk of type 2 diabetes, cardiovascular disease and other co-morbidities associated with obesity.
However, these medications do not address the underlying causes of impaired GLP-1 function, such as gut dysbiosis, liver stress, chronic inflammation, toxin accumulation, and poor dietary patterns. If these root causes are not addressed through nutrition and lifestyle interventions, stopping the medication may allow the underlying issues to resurface, potentially leading to a return of symptoms or worsening metabolic health.
Bottom line: Pairing GLP-1 medications with supportive nutrition and lifestyle changes (to address the root cause) may help improve long-term results and lower the risk of regaining weight after treatment.
Potential risks of taking GLP-1 medications
So, if the drugs are so effective, why not just go ahead and take them? Below is a summary of some of the potential side effects of the drugs, and why caution is advised when considering using them.
1. Digestive System side effects
Gastrointestinal Effects:
Common side effects include nausea, vomiting, diarrhea, and constipation, especially in the first few weeks. These usually improve over time. GLP-1 RAs slow gastric emptying, which can rarely raise concerns about intestinal blockage or aspiration during surgery.
Gallbladder Effects:
These drugs can increase the risk of gallstones and bile duct issues, particularly with higher doses, longer use, or significant weight loss. Monitoring is advised for those with a history of gallbladder problems.
Pancreatic Effects:
Although early reports raised concerns, most studies show no significant increase in pancreatitis or pancreatic cancer. Mild enzyme elevations can occur, but serious events are rare.
Takeaway:
Digestive and gallbladder issues are the most common side effects. Serious complications are uncommon, but careful monitoring is recommended for those with gallbladder or pancreatic history.
(Kim & Yoo, 2025)
2. Hormonal Disruption - UP TO HERE***
GLP-1 drugs alter the body’s natural communication between the gut and the brain. They artificially signal fullness, but in doing so, they can disturb important hormones such as ghrelin (which signals hunger), leptin (which signals satiety), and other gut–brain peptides (Müller et al., 2022; Müller, Finan, Bloom, & D’Alessio, 2023).
Over time, this interference can disrupt normal metabolic rhythms and stress hormone balance, including cortisol regulation, which may make it harder for the body to maintain weight once the medication is discontinued (Holst & Rosenkilde, 2020; Lee, Lee, & Oh, 2023).
Bottom line: Your body’s natural appetite and stress hormone rhythms may be disrupted, and may struggle to reset when the drug is stopped.
3. Nutrient Absorption Issues & Deficiencies
When appetite is suppressed, people often eat much less, sometimes too little to meet nutritional requirements.
Studies show an increased risk of nutrient deficiencies including vitamin B12, vitamin D, iron, and calcium among GLP-1 users (Papamargaritis et al., 2022).
Clinical commentary has also warned that nutrient insufficiency may underlie side effects such as fatigue, brittle bones, or the accelerated facial aging described in the media as ‘Ozempic face’ (Manne-Goehler & Franco, 2025).
Bottom line: When using these medications, it is essential to maintain an energy-balanced, nutrient-dense diet. Without this, deficiencies are likely to develop.
4. Muscle Wastage & Strength Loss
Weight lost on GLP-1 medications is not just fat, but studies have shown 20–40% may be lean muscle mass (Rosenstock et al., 2021; Wilding et al., 2021; Rubino et al., 2021).
Loss of muscle reduces metabolism, increases frailty, and impairs mobility, especially in older adults (Batsis & Villareal, 2018; Chen et al., 2020).
Research highlights that this muscle loss not only makes weight regain more likely, but also diminishes long-term strength and function (Garvey et al., 2022; Heymsfield et al., 2021).
Additionally, appetite suppression may place the body in a mild stress state. Cortisol and other stress hormones can rise when the body perceives an “energy deficit,” which may further contribute to muscle breakdown, fatigue, or lowered immunity (Ravussin & Ryan, 2018).
Bottom line: Without structured resistance training and adequate caloric/nutritional intake, patients risk sacrificing strength for short-term weight reduction.
5. Weight Regain
Because of metabolic adaptation, muscle loss, and the fact that underlying dysfunctional eating behaviours may not get addressed, weight regain is very common after stopping GLP-1 medications.
Lowered resting energy expenditure following GLP-1 cessation contributes to increased risk of weight regain, even when lifestyle support continues (Naso & Rondanelli, 2017).
Several clinical analyses confirm that weight regain after stopping GLP-1 medications is both common and sometimes rapid, substantially undermining metabolic gains (Wilding et al., 2022; Rubino et al., 2021).
Addressing eating behaviours, stress, and lifestyle patterns is therefore essential. Many practitioners recommend combining medical weight-loss therapies with counselling, behavioural therapy, or coaching to build long-term skills and resilience around food and lifestyle habits.
Bottom line: Without addressing the underlying relationship with food and lifestyle, weight regain is highly likely once GLP-1 medications are stopped. It might be advisable to combine medical treatment with counselling or behavioural therapy to support lasting transformation.
6. Toxic Load from Rapid Fat Loss
Fat tissue acts as a storage site for fat-soluble pollutants and toxins (Heindel et al., 2017). When fat breaks down quickly, these compounds are released into circulation.
This may temporarily overwhelm the liver, kidneys, and other detoxification pathways. If elimination is further impaired (e.g., by constipation), toxins may redistribute to other organs, creating additional health risks (Neeland et al., 2019).
Bottom line: Weight loss should ideally be gradual and supported with nutritional and detoxification strategies.
7. Thyroid Cancer Risk
Animal studies raised concern about thyroid tumours in response to GLP-1 drugs. Human data remain mixed and inconclusive.
A large Scandinavian study found no increased thyroid cancer risk (Pottegård et al., 2025).
A French cohort reported a 58% higher risk after 1–3 years of use (Bezin et al., 2023).
Bottom line: Following the principle of “first, do no harm,” caution is warranted until definitive, long-term data is made available.
8. Other Side Effects Reported in the Literature
Beyond those already discussed in detail above, GLP-1 receptor agonists have been associated with a wide range of additional side effects:
Common: Headache, fatigue, dizziness, injection-site reactions, mild hypoglycaemia (when combined with insulin or sulfonylureas).
Gastrointestinal: Delayed gastric emptying (problematic during surgery or anesthesia), gastroesophageal reflux, abdominal pain.
Hepatobiliary: Gallbladder disease, gallstones, biliary colic.
Pancreatic: Rare cases of pancreatitis, pancreatic enzyme elevations.
Renal: Dehydration and acute kidney injury (often secondary to vomiting or diarrhea).
Cardiovascular: Mild increases in heart rate, rare arrhythmias.
Neurological: Rare reports of altered taste, mild cognitive complaints.
Immunological: Rare hypersensitivity reactions.
Emerging / Theoretical: Alterations to the gut microbiome, long-term sarcopenia risk, and possible psychiatric side effects such as increased anxiety or depression in sensitive individuals.
(Manne-Goehler & Franco, 2025)
The Naturopathic “Do No Harm” Approach
In naturopathic medicine, primum non nocere—“first, do no harm”—means beginning with the safest, least invasive options before turning to stronger interventions. When it comes to GLP-1 medications, this principle guides us to carefully investigate the root causes of weight gain and to screen for potential risks before a person starts taking the GLP-1 drug.
Identifying Underlying Issues That Could Be Resolved Without Medication
Many drivers of weight gain can be uncovered and addressed through targeted testing, often reducing or even removing the need for pharmaceutical support:
Basic blood work (CBC, liver and kidney function): Screens for inflammation, hidden organ stress, or deficiencies that may affect metabolism and energy (Calder et al., 2017; McCullough et al., 2020).
Blood sugar regulation (fasting glucose, HbA1c, fasting insulin, HOMA-IR): Detects insulin resistance or prediabetes, which can sometimes be corrected with nutrition, exercise, and stress support (Cornier et al., 2011; Garber et al., 2020).
Thyroid panel (TSH, Free T4, Free T3, ± antibodies): Evaluates whether thyroid dysfunction is contributing to weight changes (Taylor et al., 2018).
Hormone panel (cortisol, estrogen, progesterone, testosterone, DHEA): Identifies imbalances in stress or sex hormones that may drive weight gain or resistance to fat loss (Duntas & Biondi, 2013; Pasquali, 2012).
Gut health testing (stool analysis, SIBO breath test, gut inflammation markers): Assesses digestive function, nutrient absorption, and microbiome balance, all of which affect weight and cravings (Zmora et al., 2019; Sonnenburg & Sonnenburg, 2019).
Body composition analysis (lean mass vs. fat mass): Helps distinguish fat-related weight gain from muscle loss or sarcopenia, guiding a more tailored approach (Villareal et al., 2017; Prado & Heymsfield, 2014).
Screening for Risk Factors Before Starting GLP-1 Medication
Functional and standard tests can identify whether a patient carries higher risk for known drug side effects, allowing precautions:
Gallbladder and liver markers (liver enzymes, ultrasound if indicated): Since GLP-1 drugs can increase risk of gallstones and gallbladder disease, pre-existing issues should be ruled out (Wilding et al., 2021; Nauck et al., 2016).
Pancreatic enzymes (amylase, lipase): Screens for silent pancreatic stress, important given the risk of pancreatitis with GLP-1s (Nauck et al., 2016).
Full thyroid panel ± calcitonin: Ensures baseline thyroid health and checks for red flags, since concerns exist around thyroid tumour risk (Campbell et al., 2021).
Lipid profile (cholesterol, triglycerides): Evaluates cardiovascular risk, which may influence whether these medications are the safest option (Garber et al., 2020).
Nutrient status (vitamin B12, vitamin D, iron, calcium): Identifies deficiencies that could be worsened by appetite suppression on the medication (Mullin et al., 2020; Thakkar et al., 2015).
This personalised assessment honours the naturopathic principles of:
First, do no harm – start with the safest, least invasive strategies.
Identify and treat the cause – uncover and address the true drivers of weight gain.
Support the whole person – recognise that weight is not just about calories, but about the interconnected balance of hormones, organs, and lifestyle (Cornier et al., 2011).
Solutions: Evidence-Based, Drug-Sparing Ways to Improve Insulin Sensitivity and Lose Fat
In light of all of the risks associated with the medication, what are the other options? Are there actually solutions to resolving the ROOT CAUSE of blood sugar dysregulation, excess fat storage and insatiable hunger? There sure are!
1) Make plants the foundation (especially lower-fat, higher-fibre)
A plant-predominant, lower-fat dietary pattern consistently improves insulin sensitivity, glycemic control, and cardiometabolic risk, often beyond what weight change alone would predict. Randomised trials of low-fat vegan or plant-forward diets show improved insulin sensitivity, β-cell function, and HbA1c compared with conventional diets, alongside favourable changes in body composition (Kahleová et al., 2018; Kahleová et al., 2020). High-fiber, minimally processed plant foods also nourish a healthier, more diverse gut microbiome, which is linked to lower inflammatory tone and better metabolic regulation (Singh et al., 2017; Sonnenburg & Sonnenburg, 2019).
Practical takeaways: Build meals around fruits, vegetables, legumes, whole grains and minimal nuts/seeds; keep added fats and ultra-processed foods low; aim for ≥30–40 g/day of fiber (Kahleová et al., 2020; Sonnenburg & Sonnenburg, 2019).
2) Train your metabolism with movement (resistance + aerobic)
Exercise increases insulin sensitivity through muscle-specific mechanisms even in the absence of major weight loss (Bird & Hawley, 2017). Combining resistance training with aerobic work optimizes fat loss while preserving (or gaining) lean mass, critical for sustaining resting energy expenditure and preventing the “regain” trap (Villareal et al., 2017). In older adults with obesity, programs that include both resistance and aerobic training during weight reduction improve insulin sensitivity indices, and reduce visceral and inter-muscular fat compared with either mode alone (Weiss et al., 2022).
Practical takeaways: 2–3 non-consecutive days/week of resistance training (large compound movements) plus 150–300 minutes/week of moderate aerobic activity or interval work; prioritise progressive overload and adequate recovery (Weiss et al., 2022; Bird & Hawley, 2017).
3) Address stress and emotional drivers (mindfulness, CBT, and coaching)
Stress reactivity and dysregulated eating patterns undermine metabolic health. Randomised trials and meta-analyses show mindfulness-based interventions and related programs produce small-to-moderate improvements in emotional eating and modest but meaningful weight and waist reductions, with reductions in cortisol reactivity in some studies (Daubenmier et al., 2011; Carbone et al., 2022; Rogers et al., 2021; Mason et al., 2016).
Practical takeaways: Incorporate mindfulness-based stress reduction, mindful eating skills, or CBT-based coaching alongside nutrition and exercise to lock in behaviour change and protect against relapse (Rogers et al., 2021; Mason et al., 2016).
4) Hydrate strategically
Simple hydration habits can support appetite regulation and weight control. In a randomized trial, drinking ~500 mL water before each main meal enhanced 12-week weight loss beyond a hypocaloric diet alone in middle-aged and older adults (Dennis et al., 2010). Meta-analyses now support that pre-meal water loading can significantly augment weight loss and reduce daily energy intake (Pan et al., 2021).
Practical takeaways: Target regular water intake across the day and consider a pre-meal water “preload,” particularly if you’re middle-aged or older (Dennis et al., 2010; Pan et al., 2021).
5) Guard your circadian rhythm (sleep and morning light)
Short sleep and circadian misalignment impair insulin sensitivity and promote preferential visceral fat gain, even over just a few weeks (Eckel et al., 2015; Covassin et al., 2022; Leproult & Van Cauter, 2010). Morning outdoor light helps anchor circadian timing, indirectly supporting appetite and glucose regulation (Depner et al., 2018).
Practical takeaways: Aim for 7–9 hours/night, keep a consistent sleep-wake schedule, seek outdoor morning light exposure, and limit late-evening light, heavy meals, and alcohol (Covassin et al., 2022; Depner et al., 2018).
Bottom line
A plant-focussed, lower-fat, higher-fiber diet, structured resistance + aerobic training, stress-management skills (mindfulness/CBT), consistent hydration and circadian-aligned sleep/light exposure can form a potent, drug-sparing strategy to improve insulin sensitivity, reduce visceral fat, and sustain weight loss, without the risk of medications.
These interventions directly target the biological systems GLP-1 agonists attempt to influence whilst building durable habits and metabolic resilience (Kahleová et al., 2018; Kahleová et al., 2020; Villareal et al., 2017; Weiss et al., 2022; Bird & Hawley, 2017; Rogers et al., 2021; Mason et al., 2016; Pan et al., 2021; Depner et al., 2018).
Summary
GLP-1 agonists can deliver quick results, with far less effort than changing your diet, lifestyle, exercising, getting adequate sleep and taking the time to educate yourself on your health. I totally get it - we all want a quick fix, and this drug seems to do it exceptionally well. However, if you are concerned about the risks, or potential weight regain after drug cessation, there ARE solutions out there, and other benefits to incorporating these practices into your lifestyle, such as increased energy, bowel health, balanced mood, reduced stress and increased muscle mass. The research is clear!
These drugs are not a long-lasting solution, especially if you start running into dramas with nutritional deficiencies, muscle mass loss and other side effects. There is not, and will never be any drug on the market that will RESOLVE the root cause of your health challenges. Only you can go on that journey, one day at a time.
If you’re considering these medications, I encourage you to explore thorough testing, emotional support, and nutritional strategies before or alongside your treatment. As a practitioner, there are circumstances in which I feel it would be suitable for people to take these medications for a short period of time, alongside the above natural strategies to complement the treatment. There are other times when I feel the risk factors may outweigh the benefits for some people, but this would only be discovered upon further consultation and testing.
Having said all of that, for long lasting, sustainable weight loss, I highly recommend seeking a clinically trained health practitioner to educate you, source appropriate testing, and support you on your journey. Please reach out if you’re looking for further support on this matter.
References
Australian Institute of Health and Welfare. (2023a). Diabetes: Australian facts. https://www.aihw.gov.au/reports/diabetes/diabetes/contents/summary
Australian Institute of Health and Welfare. (2023b). Overweight and obesity - Australian adults. https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity/contents/summary
Batsis, J. A., & Villareal, D. T. (2018). Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nature Reviews Endocrinology, 14(9), 513–537. https://doi.org/10.1038/s41574-018-0062-9
Bharucha, A. E., & Camilleri, M. (2021). Epidemiology and pathophysiology of chronic constipation. Gastroenterology, 160(1), 36–52. https://doi.org/10.1053/j.gastro.2020.05.078
Bird, S. R., & Hawley, J. A. (2017). Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport & Exercise Medicine, 3(1), e000143. https://doi.org/10.1136/bmjsem-2016-000143
Bezin, J., et al. (2023). GLP-1 receptor agonists and risk of thyroid cancer: A French nationwide cohort study. Diabetes Care, 46(6), 1235–1243. https://doi.org/10.2337/dc22-1923
Calder, P. C., Carr, A. C., Gombart, A. F., & Eggersdorfer, M. (2017). Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients, 12(4), 1181. https://doi.org/10.3390/nu12041181
Campbell, K., Paterson, A., & Morris, J. (2021). Thyroid health and risk assessment prior to pharmacological interventions. Endocrinology, Diabetes & Metabolism Case Reports, 2021, 21–0351. https://doi.org/10.1530/EDM-21-0351
Camilleri, M., & Katzka, D. A. (2012). Motor functions of the intestine. Schiff’s Diseases of the Liver, 2(11), 207–216. https://doi.org/10.1002/9781119950509.ch11
Carbone, E., Tully, A., & Smith, E. (2022). Mindfulness and cognitive behavioral interventions for emotional eating: A systematic review. Nutrients, 14(9), 1802. https://doi.org/10.3390/nu14091802
Chen, L. K., Woo, J., Assantachai, P., et al. (2020). Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. Journal of the American Medical Directors Association, 21(3), 300–307.e2. https://doi.org/10.1016/j.jamda.2019.12.012
Cornier, M. A., Després, J.-P., Davis, N., Grossniklaus, D. A., Klein, S., Lamarche, B., … & Eckel, R. H. (2011). Assessing adiposity: A scientific statement from the American Heart Association. Circulation, 124(18), 1996–2019. https://doi.org/10.1161/CIR.0b013e318233bc6a
Covassin, N., Singh, P., & Staley, K. (2022). Sleep, circadian rhythms, and metabolic health: A review of the evidence. Current Opinion in Endocrinology, Diabetes and Obesity, 29(4), 269–277. https://doi.org/10.1097/MED.0000000000000704
Cusi, K. (2016). NAFLD and type 2 diabetes: Pathophysiological mechanisms and treatment strategies. Hepatology, 64(1), 149–162. https://doi.org/10.1002/hep.28428
Daubenmier, J., Hayden, D., Chang, V., Epel, E., & Kristeller, J. (2011). Impact of a mindfulness-based intervention on distress eating and glucose control in overweight adults with type 2 diabetes. Obesity, 19(12), 2413–2419. https://doi.org/10.1038/oby.2011.263
Davies, M., Færch, L., Jeppesen, O. K., Pakseresht, A., Pedersen, S. D., Perreault, L., … & Rosenstock, J. (2021). Semaglutide 2.4 mg once a week in adults with overweight or obesity. New England Journal of Medicine, 384(11), 989–1002. https://doi.org/10.1056/NEJMoa2032183
Dennis, E. A., Dengo, A. L., Comber, D. L., Flack, K. D., Savla, J., Davy, K. P., & Davy, B. M. (2010). Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity, 18(2), 300–307. https://doi.org/10.1038/oby.2009.235
Depner, C. M., Stothard, E. R., & Wright, K. P. Jr. (2018). Metabolic consequences of sleep and circadian disorders. Current Diabetes Reports, 18, 67. https://doi.org/10.1007/s11892-018-1029-9
Depommier, C., Everard, A., Druart, C., Plovier, H., Van Hul, M., Vieira-Silva, S., ... & Cani, P. D. (2019). Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nature Medicine, 25, 1096–1103. https://doi.org/10.1038/s41591-019-0495-2
Diabetes Australia. (2023). Diabetes in Australia: Statistics and facts. https://www.diabetesaustralia.com.au/about-diabetes/facts-statistics
Donath, M. Y. (2014). Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nature Reviews Drug Discovery, 13, 465–476. https://doi.org/10.1038/nrd4309
Duntas, L. H., & Biondi, B. (2013). The interconnections between thyroid and obesity. The Journal of Clinical Endocrinology & Metabolism, 98(4), 1418–1425. https://doi.org/10.1210/jc.2012-3963 SCIRP
Eckel, R. H., Depner, C. M., Perreault, L., Markwald, R. R., Smith, M. R., McHill, A. W., Higgins, J., Melanson, E. L., & Wright, K. P., Jr. (2015). Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Current Biology, 25(22), 3004–3010. https://doi.org/10.1016/j.cub.2015.10.011
Everard, A., & Cani, P. D. (2013). Diabetes, obesity and the gut microbiota: The role of GLP-1. Gut, 62(8), 1165–1167. https://www.sciencedirect.com/science/article/pii/S1521691813000619?via%3Dihub
Fan, J., Song, H., Wang, Y., & Ding, Q. (2021). GLP-1 receptor agonists and non-alcoholic fatty liver disease: A review of mechanisms and clinical evidence. Frontiers in Endocrinology, 12, 692103. https://doi.org/10.3389/fendo.2021.692103
Garber, A. J., Handelsman, Y., Einhorn, D., Bergman, D., Bloomgarden, Z. T., Bonow, R. O., … & Umpierrez, G. (2020). Diagnosis and management of prediabetes: A review. JAMA, 323(10), 951–963. https://doi.org/10.1001/jama.2020.1401
Garvey, W. T., et al. (2022). Weight loss and muscle preservation with anti-obesity pharmacotherapy. Obesity, 30(4), 789–798. https://doi.org/10.1002/oby.23471
Ghosal, S., Xu, D., Sanchez-Garrido, M. A., Ma, Y., Thomas, D. R., Shin, M., ... & D’Alessio, D. A. (2023). Stress-induced alterations in GLP-1 signaling: Implications for glucose homeostasis and appetite. Cell Metabolism, 35(2), 250–265. https://doi.org/10.1016/j.cmet.2023.01.005
Gregor, M. F., & Hotamisligil, G. S. (2011). Inflammatory mechanisms in obesity. Annual Review of Immunology, 29, 415–445. https://doi.org/10.1146/annurev-immunol-031210-101322
Heindel, J. J., Blumberg, B., & Cave, M. (2017). Metabolism disrupting chemicals and metabolic health. Environmental Health Perspectives, 125(6), 064001. https://doi.org/10.1289/EHP1863
Heindel, J. J., et al. (2017). Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology, 68, 3–33. https://doi.org/10.1016/j.reprotox.2016.10.001
Heymsfield, S. B., et al. (2021). Muscle mass, strength, and function in obesity and after weight loss. Obesity Reviews, 22(S2), e13238. https://doi.org/10.1111/obr.13238
Holst, J. J., & Rosenkilde, M. M. (2020). GIP as a therapeutic target in diabetes and obesity: Insight from incretin co-agonists. Journal of Clinical Endocrinology & Metabolism, 105(12), e2710–e2716. https://doi.org/10.1210/clinem/dgaa327
Kahleová, H., Levin, S., & Barnard, N. D. (2020). Cardio-metabolic benefits of plant-based diets. Nutrients, 12(11), 3520. https://doi.org/10.3390/nu12113520
Kahleová, H., Pelikanová, T., & Barnard, N. D. (2018). Vegetarian diets in the prevention and treatment of type 2 diabetes. Journal of the Geriatric Cardiology, 15(7), 578–586. https://doi.org/10.11909/j.issn.1671-5411.2018.07.007
Kahleová, H., Tura, A., Hill, M., Holubkov, R., & Barnard, N. D. (2017). A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: A randomized clinical trial. Nutrition & Diabetes, 7(6), e283. https://doi.org/10.1038/nutd.2017.24
Kushner, R. F., Calanna, S., Davies, M., Dicker, D., Garvey, W. T., Goldman, B., … & Rubino, D. (2020). Clinical characteristics and outcomes associated with weight loss in patients treated with semaglutide. Diabetes, Obesity and Metabolism, 22(2), 1742–1751. https://doi.org/10.1111/dom.14034
Lee, Y., Lee, H., & Oh, T. J. (2023). The role of incretin hormones in stress, energy homeostasis, and weight regulation. Frontiers in Endocrinology, 14, 1132451. https://doi.org/10.3389/fendo.2023.1132451
Leproult, R., & Van Cauter, E. (2010). Role of sleep and sleep loss in hormonal release and metabolism. Endocrine Development, 17, 11–21. https://doi.org/10.1159/000262524
Manne-Goehler, J., & Franco, J. (2025). Side effects of GLP-1 receptor agonists. The BMJ, 390, r1606. https://doi.org/10.1136/bmj.r1606BMJ
Marso, S. P., Bain, S. C., Consoli, A., Eliaschewitz, F. G., Jódar, E., Leiter, L. A., ... & Wanner, C. (2016). Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. The New England Journal of Medicine, 375(19), 1834–1844. https://doi.org/10.1056/NEJMoa1607141
Mason, A. E., Jhaveri, K., & Goldstein, S. (2016). Mindfulness-based interventions for weight loss and disordered eating: A systematic review. Obesity Reviews, 17(10), 1015–1029. https://doi.org/10.1111/obr.12450
McCullough, A., Horgan, G., & McMorrow, A. (2020). Liver function tests: Clinical and diagnostic applications. Clinical Biochemistry, 78, 1–10. https://doi.org/10.1016/j.clinbiochem.2020.03.007
Monteiro, C. A., Cannon, G., Levy, R. B., Moubarac, J. C., & Louzada, M. L. C. (2019). Ultra-processed foods: What they are and how to identify them. Public Health Nutrition, 22(5), 936–941. https://doi.org/10.1017/S1368980018003762Wikipedia+2Cambridge University Press & Assessment+2
Müller, T. D., Finan, B., Bloom, S. R., & D’Alessio, D. (2023). Glucagon-like peptide-1 (GLP-1). Molecular Metabolism, 66, 101650. https://doi.org/10.1016/j.molmet.2022.101650
Müller, T. D., Finan, B., Clemmensen, C., DiMarchi, R. D., & Tschöp, M. H. (2022). The new biology and pharmacology of glucagon-like peptide-1. Physiological Reviews, 102(4), 1565–1622. https://doi.org/10.1152/physrev.00034.2020
Mullin, G. E., Park, Y., & Barton, C. (2020). Nutritional considerations for patients with obesity and diabetes. Nutrients, 12(12), 3802. https://doi.org/10.3390/nu12123802
Nauck, M. A., Meier, J. J., Cavender, M. A., & El-Ouaghlidi, A. (2016). Pancreatic safety of GLP-1 receptor agonists. Diabetes Obesity and Metabolism, 18(8), 738–746. https://doi.org/10.1111/dom.12651
Naso, A., & Rondanelli, M. (2017). Weight regain after weight loss: Current knowledge and future directions. Current Obesity Reports, 6(3), 236–245. https://doi.org/10.1007/s13679-017-0279-8
Neeland, I. J., Ross, R., Després, J. P., Matsuzawa, Y., Yamashita, S., Shai, I., … & Després, J. P. (2019). Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes & Endocrinology, 7(9), 715–725. https://doi.org/10.1016/S2213-8587(19)30084-1
Obesity Evidence Hub. (2023). Overweight and obesity prevalence in Australia. https://www.obesityaustralia.org
Pan, A., Scherer, R., & Binns, C. (2021). Water intake and weight loss: A systematic review and meta-analysis. Nutrition Reviews, 79(7), 834–846. https://doi.org/10.1093/nutrit/nuaa137
Papamargaritis, D., et al. (2022). Nutrient deficiency risks associated with GLP-1 receptor agonists: What patients and clinicians should know. Celebrate Vitamins. Retrieved from https://celebratevitamins.com/a/blog/nutrient-deficiency-risks-associated-with-glp-1-receptor-agonists-what-patients-and-clinicians-should-knowCelebrate Vitamins
Pasquali, R. (2012). Obesity and androgens: Facts and perspectives. Frontiers of Hormone Research, 40, 63–76. https://doi.org/10.1159/000335074
Prado, C. M., & Heymsfield, S. B. (2014). Lean tissue imaging: A new era for nutritional assessment and intervention. Journal of Parenteral and Enteral Nutrition, 38(8), 940–953. https://doi.org/10.1177/0148607114550189
Pottegård, A., et al. (2025). GLP-1 receptor agonists and risk of thyroid cancer in Scandinavia: A multinational cohort study. BMJ, 380, e072785. https://doi.org/10.1136/bmj-2022-072785
Ravussin, E., & Ryan, D. H. (2018). Energy expenditure and hormonal adaptations to caloric restriction and weight loss. Nature Reviews Endocrinology, 14(1), 12–28. https://doi.org/10.1038/nrendo.2017.119
Rogers, P. J., Appleton, K. M., & Kessler, D. (2021). Mindfulness interventions for improving eating behaviours and metabolic outcomes: A review of RCT evidence. Nutrients, 13(10), 3511. https://doi.org/10.3390/nu13103511
Rosenstock, J., et al. (2021). Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine, 384(11), 989–1002. https://doi.org/10.1056/NEJMoa2032183
Rubino, D., et al. (2021). Effect of continued weekly subcutaneous semaglutide vs. placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 trial. JAMA, 325(14), 1414–1425. https://doi.org/10.1001/jama.2021.3224
Rubino, D. M., Nathan, D. M., & Cummings, D. E. (2022). The role of GLP-1 in the regulation of glucose homeostasis and appetite. Diabetes, Obesity and Metabolism, 24(1), 1–13. https://doi.org/10.1111/dom.14691
Santoro, A., Guidi, A., Saba, L., Di Giorgio, A., & Chiarelli, F. (2021). Environmental pollutants and metabolic disorders: The interplay of endocrine disruptors and gut-liver axis. Frontiers in Endocrinology, 12, 667703. https://doi.org/10.3389/fendo.2021.667703
Shin, A., & Camilleri, M. (2013). Diagnostic assessment of diabetic gastroparesis. Diabetes, 62(9), 2667–2673. https://doi.org/10.2337/db13-0482
Singh, R. K., Chang, H.-W., Yan, D., Lee, K. M., Ucmak, D., Wong, K., ... & Liao, W. (2017). Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 15, 73. https://doi.org/10.1186/s12967-017-1175-y
Sonnenburg, J. L., & Sonnenburg, E. D. (2019). The ancestral and industrialized gut microbiota and implications for human health. Nature Reviews Microbiology, 17(6), 383–390. https://doi.org/10.1038/s41579-019-0191-8
Taylor, P. N., Albrecht, D., Scholz, A., Gutierrez-Buey, G., Lazarus, J. H., Dayan, C. M., & Okosieme, O. E. (2018). Global epidemiology of hyperthyroidism and hypothyroidism. Nature Reviews Endocrinology, 14(5), 301–316. https://doi.org/10.1038/nrendo.2018.18
Thakkar, S., Sharma, D., & Sahni, S. (2015). Nutritional deficiencies in adults and the importance of monitoring. Current Opinion in Clinical Nutrition & Metabolic Care, 18(6), 507–513. https://doi.org/10.1097/MCO.0000000000000210
Villareal, D. T., Aguirre, L., Gurney, A. B., Waters, D. L., & Sinacore, D. R. (2017). Aerobic or resistance exercise, or both, in dieting obese older adults. New England Journal of Medicine, 376, 1943–1955. https://doi.org/10.1056/NEJMoa1616338
Vilsbøll, T., Christensen, M., Junker, A. E., Knop, F. K., & Gluud, L. L. (2012). Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ, 344, d7771. https://doi.org/10.1136/bmj.d7771
Wilding, J. P. H., Batterham, R. L., Calanna, S., Davies, M., van Gaal, L. F., Lingvay, I., ... & Wadden, T. A. (2021). Once-weekly semaglutide in adults with overweight or obesity. The New England Journal of Medicine, 384(11), 989–1002. https://doi.org/10.1056/NEJMoa2032183
Wilding, J. P. H., et al. (2022). Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes, Obesity and Metabolism, 24(8), 1553–1564. https://doi.org/10.1111/dom.14725
Weiss, E. P., Albert, S. G., & Villareal, D. T. (2022). Effects of exercise modality on insulin sensitivity and body composition in older adults. Diabetes Care, 45(4), 823–832. https://doi.org/10.2337/dc21-1403
Younossi, Z., Anstee, Q. M., Marietti, M., Hardy, T., Henry, L., Eslam, M., ... & Bugianesi, E. (2018). Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology & Hepatology, 15, 11–20. https://doi.org/10.1038/nrgastro.2017.109
Zhang, X., Lan, R., Xu, W., & Zhu, H. (2021). Gut microbiota in obesity and metabolic disorders – A review. Frontiers in Medicine, 8, 713435. https://doi.org/10.3389/fmed.2021.713435
Zmora, N., Suez, J., & Elinav, E. (2019). You are what you eat: Diet, health, and the gut microbiota. Nature Reviews Gastroenterology & Hepatology, 16(1), 35–56. https://doi.org/10.1038/s41575-018-0061-2